Abstract
Dasatinib with corticosteroid yields high complete remission (CR) rates in Ph+ ALL with minimal induction death. Optimal post-remission therapy is not known. We report a prospective study evaluating dasatinib/dexamethasone induction then consolidation with reduced-intensity conditioning (RIC) allogeneic hematopoietic cell transplantation (alloHCT), autologous HCT (autoHCT), or chemotherapy alone (chemo), followed by dasatinib-based maintenance.
Methods:
We conducted a phase II, 3-arm, non-randomized trial (NCT01256398, Support: U10CA180821, U10CA180882, U10CA180861, U24CA196171) at 17 US centers with primary objectives to determine 3-year overall survival (OS) and disease-free survival (DFS) of adults ≥18 years (yrs) old with untreated Ph+ ALL treated with dasatinib/dexamethasone induction, CNS prophylaxis with systemic and intrathecal methotrexate, consolidation with alloHCT, autoHCT, or chemo, then dasatinib maintenance. Induction (Course I) used dasatinib 140 mg orally daily and dexamethasone 10 mg/m2/day orally or IV days 1-7. If ≤20% lymphoblasts in marrow at Day 15, Course II continued dasatinib with 7 days of dexamethasone. If >20% lymphoblasts, patients (pts) also received vincristine and daunorubicin. Pts not in CR/CRi received a second induction (Course III) with dasatinib, cyclophosphamide, vincristine, daunorubicin, and dexamethasone. CNS prophylaxis (Course IV) used dasatinib, IV vincristine and IV, oral, and intrathecal methotrexate. Course V consisted of HCT or chemo. Pts 18-70 yrs old underwent RIC alloHCT if they had a HLA-matched donor; otherwise they underwent autoHCT. AlloHCT conditioning used fludarabine 30 mg/m2/day IV day -7 through -3, alemtuzumab 20 mg/day IV day -7 through -3, and melphalan 140 mg/m2 IV once on day -2. GVHD prophylaxis with tacrolimus began day -2. Pts undergoing autoHCT received etoposide 10 mg/kg/day (age >65, 5 mg/kg/day) CIV for 4 days and cytarabine 2 g/m2 (age >65, 1 g/m2) IV every 12 hours for 8 doses then G-CSF for mobilization. AutoHCT conditioning was melphalan 100 mg/m2/day IV on days -2 and -1. Pts >70 yrs old or unable to undergo HCT received etoposide/cytarabine alone. Dasatinib maintenance (Course VI) began day 30 of Course V and continued for 12 months and until 2 consecutive negative BCR-ABL1 RT-PCR assays 3 months apart or relapse. BCR-ABL1 isoform and ABL1 kinase domain (KD) mutation determination were done locally.
Results:
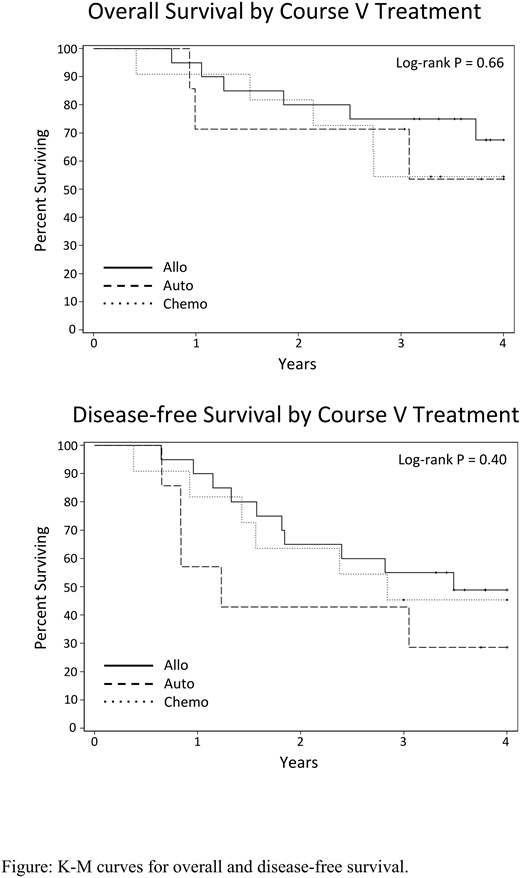
From 12/15/2010 to 11/14/2014, 64 eligible pts enrolled. Median age was 60 yrs (22-87); median WBC 23.2 x 103/μl (0.3-454). The dominant BCR-ABL1 isoform was p190 in 59%, p210 in 17%, and not reported in 23%. The CR rate was 97% with no induction deaths. Fifty-five pts completed CNS prophylaxis (Course IV). Twenty pts underwent protocol-specified alloHCT, 7 autoHCT, and 9 chemo. Nine pts underwent off-study alloHCT and 5 skipped Course V. Dasatinib maintenance was tolerable with 72%, 80%, and 80% receiving >50% of planned maintenance doses in alloHCT, autoHCT, and chemo arms, respectively. With a median follow up of 48 months (37-78) for survivors, 3-yr OS and DFS were 55% (median OS 45 months) and 43% (median DFS 30 months), respectively. For pts undergoing consolidation with protocol-specified alloHCT, autoHCT, or chemo, 3-yr OS was 75%, 71%, and 55% respectively with median OS not reached (NR) for all groups. DFS at 3-yrs was 55%, 43%, and 46% respectively. Median DFS for alloHCT was 42 months vs 15 months for autoHCT and 34 months for chemo (Log-Rank P=0.4). Outcomes were similar with inclusion of off-study alloHCT and chemo patients skipping Course V. Relative to p190 BCR-ABL1 isoform, p210 was associated with shorter median OS (NR vs 16 months, P=0.04) and median DFS (35 months vs 10 months, P<0.0001). Three-year DFS was 9% with p210. Relapse occurred in 23 pts with 5 CNS relapses reported, one isolated. Relapse occurred in 25% of alloHCT, 43% of autoHCT, and 37% of chemo pts. T315I mutation in ABL1 KD was detected in 6 of 8 marrow relapses (75%). One isolated CNS relapse had no ABL1 KD mutations.
Conclusions:
Dasatinib and dexamethasone followed by alloHCT, autoHCT, or chemo yield similar 3-yr survival comparable to approaches using intensive induction chemotherapy. Pts with the p210 BCR-ABL1 isoform had dismal outcomes and may benefit from alternate approaches. T315I mutation was the major cause of relapse and should be addressed in future studies.
Wieduwilt:Amgen: Research Funding; Reata Pharmaceuticals: Equity Ownership; Daiichi Sankyo: Membership on an entity's Board of Directors or advisory committees; Leadiant: Research Funding; Shire: Research Funding; Merck: Research Funding. Uy:GlycoMimetics: Consultancy; Curis: Consultancy. Powell:Rafael Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees. Kolitz:Magellan Health: Consultancy, Honoraria. Liedtke:Caelum: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen/Onyx: Consultancy, Honoraria, Research Funding; Gilead: Membership on an entity's Board of Directors or advisory committees, Research Funding; celgene: Research Funding; Genentech/Roche: Research Funding; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Prothena: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BlueBirdBio: Research Funding. Stock:Jazz Pharmaceuticals: Consultancy. Devine:Kiadis Pharma: Consultancy. Larson:BristolMyers Squibb: Consultancy, Research Funding; Ariad/Takeda: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal